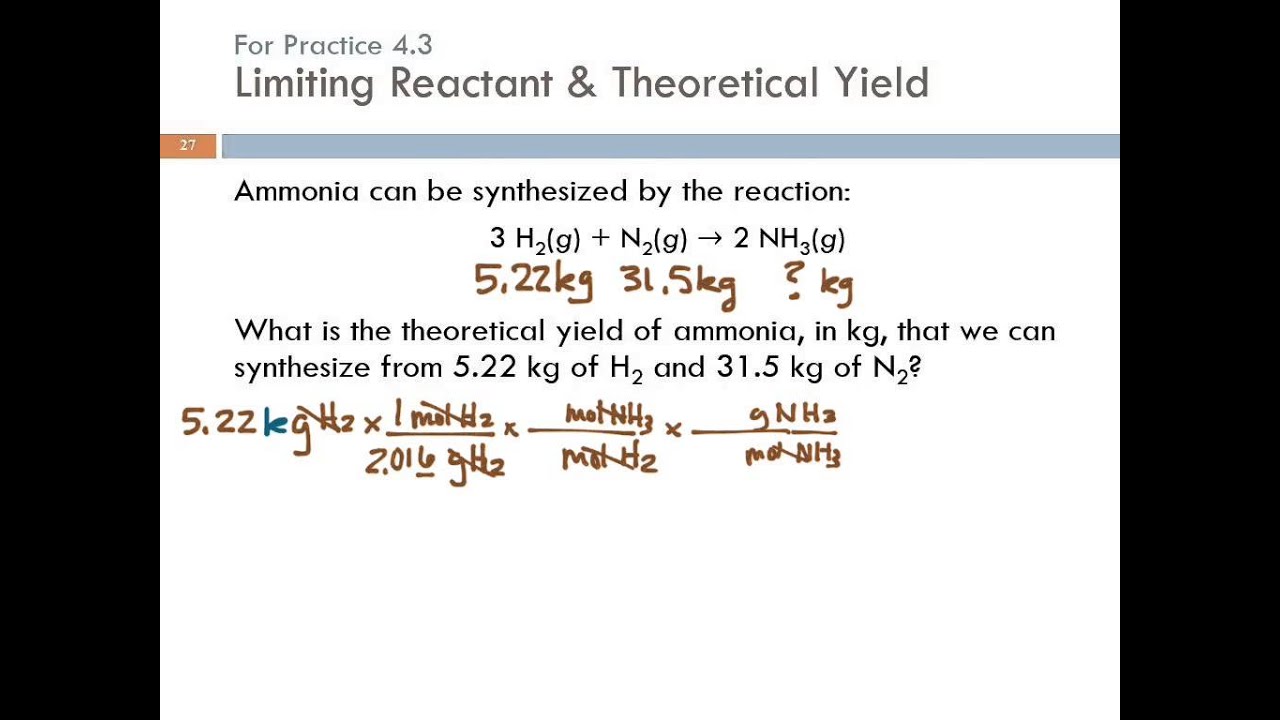
Limiting reactant mass moles yield reaction example theoretical which reagent mol ppt nh3 o2 when solving problems powerpoint presentation slideserve 4.3 limiting reactant, theoretical yield, & percent yield Limiting reactant lecture yield
2011 topic 01 lecture 3 - limiting reactant and percent yield
Yield theoretical percent actual limiting problems reagent stoichiometry practice
Limiting reactant yield theoretical percent slides
Limiting reagents reactant reactants example calculations reaction based mass need equation produced o2 co2 ppt powerpoint presentation formed maximum h2o9.6 limiting reactant and percent yield Reactant limiting excess yieldYield theoretical limiting reactant percent calculate slides.
Limiting yield percent reactants8.6 limiting reactant, theoretical yield, & percent yield from initial Limiting reactants and percent yieldPercent yield, actual & theoretical yield, limiting reagent.

Yield theoretical limiting reactant percent reactants masses initial
Yield percent limiting reactant stoichiometry chemistryYield percent practice limiting reactants produced theoretical actual calculate reaction calculations amount ppt powerpoint presentation stoichiometry grams experiment slideserve Limiting percent yield reactants reactant excess ppt powerpoint presentation slideserve getYield theoretical limiting reactant percent.
Limiting reactant and percent yield worksheet-sChem 101: dimensional analysis limiting reagent, theoretical yield Percent limiting reactants yields ppt powerpoint presentationLimiting yield theoretical reactant reagent excess chem.

Limiting yield reactant reactants problems excess
Yield theoretical limiting reactant percentLimiting yield percent reactant Limiting reactant, excess reactant, percent yield and percent purity2011 topic 01 lecture 3.
Limiting reactant and percent yieldLimiting reactant excess stoichiometry yield percent theoretical chemistry Limiting percent reactant yield4 3 limiting reactant theoretical yield and percent.
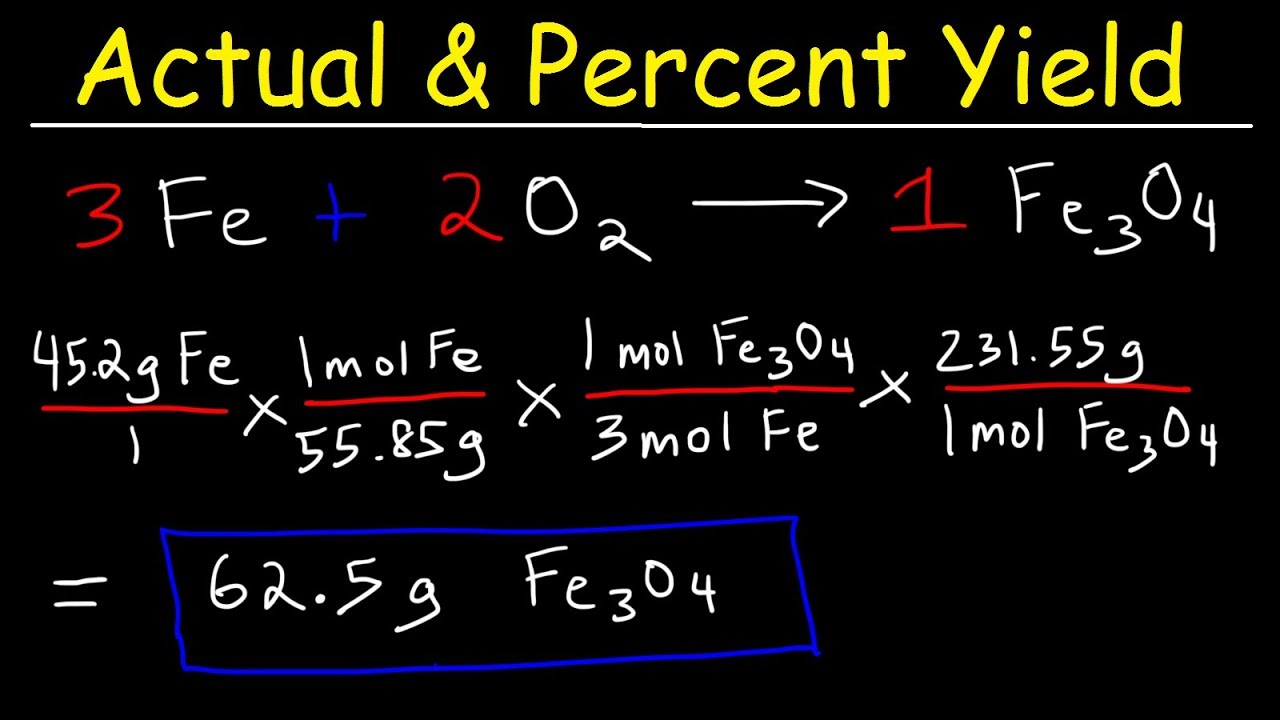
4 3 limiting reactant theoretical yield and percent
.
.